Abstract
This study aimed to evaluate the efficacy of 3 mouthwashes in reducing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load in the saliva of coronavirus disease 2019 (COVID-19) patients at 30 min, 1, 2 and 3 h after rinsing. This pilot study included 40 admitted COVID-19 positive patients (10 in each group). Saliva samples were collected before rinsing and at 30 min, 1, 2 and 3 h after rinsing with: Group 1—0.2% Chlorhexidine digluconate (CHX); Group 2—1.5% Hydrogen peroxide (H2O2); Group 3—Cetylpyridinium chloride (CPC) or Group 4 (control group)—No rinsing. Viral load analysis of saliva samples was assessed by Reverse Transcription quantitative PCR. Mean log10 viral load at different time points was compared to that at baseline in all groups using a random effects linear regression analysis while for comparison between groups linear regression analysis was used. The results showed that all groups had a significantly reduced mean log10 viral load both at 2 (p = 0.036) and 3 (p = 0.041) hours compared to baseline. However, there was no difference in mean log10 viral load between any of the investigated mouthwashes and the control group (non-rinsing) at the evaluated time points. Although a reduction in the SARS-CoV-2 viral load in the saliva of COVID-19 patients was observed after rinsing with mouthwashes containing 0.2% CHX, 1.5% H2O2, or CPC, the reduction detected was similar to that achieved by the control group at the investigated time points. The findings of this study may suggest that the mechanical action of rinsing/spitting results in reduction of SARS-CoV-2 salivary load.
Introduction
Coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also commonly known as coronavirus, was declared a pandemic in 2020 by the World Health Organization (WHO) presenting with more than 500 million confirmed cases and 6 million deaths worldwide1. COVID-19 is characterized by an unpredictable disease course, ranging from asymptomatic to severe, life-threatening infections2. SARS-CoV-2, part of a group of ‘enveloped viruses’ characterized by an outer lipid membrane3, has been detected in various clinical specimens such as saliva, throat, nasopharyngeal (NPS), and oropharyngeal (OPS) swabs, and bronchoalveolar-lavage fluid4. Angiotensin-converting enzyme II (ACE2), a cell receptor for SARS-CoV which plays an important role in the entry of the virus into the cell, is highly expressed in the oral cavity and oral epithelial cells5. A study by To et al.6 demonstrated SARS-CoV-2 being detected in 91.7% of the saliva samples obtained from COVID-19 positive patients. In addition, a recent study has further shown that detection rate of SARS-CoV-2 virus in saliva samples can be even higher than that on NPS (93.1% versus 52.5%)7. Therefore, saliva from asymptomatic or symptomatic infected subjects could be considered a high-risk route for SARS-CoV-2 infection8.
Most measures initially adopted on preventing and limiting transmission of the virus focused on performing good respiratory and hand hygiene, maintaining physical distance, wearing facial masks, and self-isolating. Despite that, different approaches have been proposed as viricidal strategies to target coronaviruses and to interfere with the viral lipid envelope3,9,10. Previous studies have suggested that constituents present in oral hygiene products such as toothpastes11 and mouthwashes might disrupt the envelope of the virus, an antiviral activity that could inactivate SARS-CoV-2 and potentially dampen transmission of virus3,10,12. A recent study investigated the short-term effects of brushing with different toothpastes on the SARS-CoV-2 salivary viral load of patients with COVID-19 showing that immediately after brushing, the use of antimicrobial toothpastes reduced the SARS-CoV-2 salivary viral load11.
Mouthwashes have been widely advocated as an adjunctive treatment to mechanical oral hygiene to reduce carious lesions, formation of dental biofilm and gingivitis13. Moreover, they have a high level of acceptance among the public due to their ease of use and breath-freshening effect. Different types of mouthwashes exist according to their active ingredients such as cetylpyridinium chloride (CPC), essential oils, chlorhexidine (CHX) or triclosan13. Experimental and clinical studies on viral infections have shown that the use of antimicrobial mouthwashes containing povidone-iodine14, chlorhexidine gluconate15,16, and cetylpyridinium chloride17 could reduce viral load. Furthermore, recently it has been demonstrated that the use of CHX, CPC, essential oils or povidone-iodine mouthwashes may reduce SARS-CoV-2 viral load in saliva16,18,19. CHX mouthwashes are broadly used as an adjunctive therapy to mechanical plaque removal to reduce oral microorganisms and to prevent oral infections. A report on its in vitro viricidal effectiveness at a concertation of 0.12% showed it may decrease the viral load of enveloped viruses15. A recent case study with 2 patients evaluated the viral dynamics in various body fluid specimens including saliva of patients with COVID-1916. The findings showed that CHX mouthwash reduced SARS-CoV-2 viral load in the patient’s saliva for 2 h after using the mouthwash, but it increased again at 2–4 h post-mouthwash use16. Hydrogen peroxide (H2O2) formulations have also been suggested to present antiviral activity that could destroy the outer layer of viruses3,9,10. Further antimicrobial agents, such as CPC have demonstrated a viricidal activity against susceptible and resistant strains of enveloped viruses by targeting and disrupting the viral envelope. A recent in vitro study assessing the viricidal activity of four mouthwashes demonstrated that the mouthwash formulated with 0.07% CPC showed viricidal effects providing a reduction of Human Coronavirus Strain (HCoV-229E) viral count18. In addition, a randomized clinical trial has shown that 0.075% CPC formulated commercial mouth rinse could decrease salivary SARS-CoV-2 levels20.
Despite the current findings and promising results of early trials, clinical information regarding efficacy of mouthwashes containing substances such as CHX, H2O2, and CPC in reducing viral load in the saliva of COVID-19 positive patients and its effects on various SARS-CoV-2 strains is still scarce and contradictory21,22,23,24,25. This pilot study tested the hypothesis that mouth rinsing with one of the investigated mouthwashes (0.2% CHX, 1.5% H2O2 or CPC) would reduce SARS-CoV-2 viral load in the saliva of COVID-19 positive patients at different time points compared to baseline; and that SARS-CoV-2 viral load at each study time point, would differ between rinsing and no rinsing groups. Therefore, the primary objective was to evaluate the efficacy of 3 different antimicrobial mouthwashes containing 0.2% Chlorhexidine digluconate, 1.5% Hydroxide peroxide and Cetylpyridinium chloride in reducing SARS-CoV-2 viral load in the saliva of hospitalized COVID-19 positive patients at 30 min, 1, 2 and 3 h after rinsing. The secondary objective aimed to compare SARS-CoV-2 viral load in the saliva of COVID-19 positive patients, at different time points, between the 3 mouthwashes (test) and no rinsing (control) groups.
Materials and methods
This was a pilot study conducted in full accordance with the ethical principles of Declaration of Helsinki, revised in 2013 and Good Clinical Practice (GCP) Guidelines. The study protocol was independently reviewed and approved by the National Health Service (NHS) Solihull Research Ethics Committee (Reference number 21/WM/0068; IRAS Number 289334; initial approval 20/04/2021) and registered in a clinical trial registry (ClinicalTrial.gov NCT04723446; 25/01/2021). The study followed the CONSORT checklist for reporting a pilot study (S1 Table).
Participants and eligibility
Patients were identified in the inpatient wards of Newham University and The Royal London Hospitals (Barts Health NHS Trust, United Kingdom) between April and October 2021, during two peaks of the pandemic and when delta was the prevalent COVID-19 strain26. Subjects were eligible for inclusion if they fulfilled the following criteria:
- Males and females, ≥ 18 years old.
- COVID-19 positive confirmed via any diagnostic test and/or presented with COVID-19 clinical symptoms at time of consent.
The exclusion criteria were:
- known pre-existing chronic mucosal lesions e.g., lichen planus or other oropharyngeal lesions, reported by patient or recorded in the existing patient’ medical notes;
- patients intubated or not capable of mouth rinsing or spitting;
- history of head and neck radiotherapy or chemotherapy;
- self-reported xerostomia;
- known allergy or hypersensitivity to chlorhexidine digluconate or any of the mouthwashes constituents;
- other severe acute or chronic medical or psychiatric condition or laboratory abnormality that could increase the risk associated with trial participation or could interfere with the interpretation of trial results and, in the judgement of the investigator, would make the subject inappropriate for entry into the trial.
- inability to comply with study protocol.
The judgment of the investigator was based on the patients’ direct care medical team opinion/recommendation. Before approaching potential participants investigators communicated with patients’ direct care medical team to understand if patient was medically stable and if their participation in the study would not worsen their condition, as well as if they were mentally and physically able to consent and comply with the study protocol.
Potential participants were then approached and provided with the study Patient Information Sheet (PIS) and received explanation about the study. The eligible patients interested in taking part in the study were then invited to sign an informed consent form. Whenever available, COVID-19 test results were confirmed before the patient entered the study. For those patients presenting COVID-19 clinical symptoms at time of consent, positive test status was confirmed within 2 weeks from the date the patient has been consented into the study.
Out of 177 inpatients initially screened, 54 fulfilled the eligibility criteria and were consented for the study. Beyond the initial sample size (n = 40) more participants were recruited into the study, as some of the patients presenting with clinical symptoms at point of consent tested negative for COVID-19 (n = 4) and some of participants initially confirmed as COVID-19 positive by diagnostic test had undetectable SARS-CoV-2 viral RNA in the baseline saliva samples (7; 14.9%). Additionally, after enrolment, 1 participant withdrew consent and 2 were withdrawn from the study by the researcher due to deteriorating medical condition. Characteristics of patients consented but not included (n = 14) in the analysis are presented in the Table S2.
In addition, up to 5 COVID-19 negative participants were recruited and provided signed informed consent as volunteers from, Barts and The London School of Medicine and Dentistry, Institute of Dentistry, Queen Mary University of London to set up the saliva profile of COVID-19 negative patients for analysis.
Study design and randomization
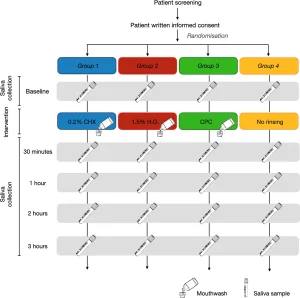
This was a single-blind, parallel-group, randomized controlled pilot study which consisted of a single study visit (Fig. 1). Allocation to intervention group took place via a balanced random permuted block approach. Sets with constant size (4-unit block size) were generated via a computer-based random number generator (http://www.randomizer.org/27), ensuring that patients were allocated in balanced blocks to one of the four groups. At the time of enrolment and after informed consent was signed, the study investigator responsible for the intervention allocated each participant as per randomisation to one of the 4 groups:
- Group 1 (test group)—0.2% Chlorhexidine digluconate (Corsodyl Alcohol free, GlaxoSmithKline, Brentford, United Kingdom).
- Group 2 (test group)—1.5% Hydrogen peroxide (Colgate Peroxyl, Colgate-Palmolive, Guildford, United Kingdom).
- Group 3 (test group)—Cetylpyridinium chloride (Oral-B Gum & Enamel Care, Procter & Gamble, Ohio, United States).
- Group 4 (control group)—No rinsing (not even water).
Medical history, oral hygiene habits and demographics
Medical history was obtained as part of the visit, including demographics, oral hygiene habits (e.g., use of mouthwashes and time of last oral hygiene procedure) and concomitant drug use information. Medical history also included relevant results of physical exam, biochemical analysis, diagnostic imaging, COVID-19 test and strain of SARS-CoV-2 variant.
Source documents consisted of patient hospital records (paper or electronic notes) as well as COVID-19 test results/certificates.
Saliva sample collection
All participants received a sealed self-test kit containing 5 self-collection saliva vials (OMNIgene Oral – OME-505, DNAgenotek, Ottawa, Canada), one for each different time point, and were given instruction to refrain from eating, drinking, chewing gum or performing oral hygiene for at least 30 minutes prior saliva collection (as per manufacturer’s instructions). Participants were then asked to collect a baseline sample of non-stimulated saliva by pooling saliva in the floor of their mouth without swallowing, and then to spit into the sterile vial until the amount of liquid saliva reached the 1 ml indication. At 30 minutes, 1, 2 and 3 hours after rinsing (test groups) or no rinsing (control group) participants were requested to collect saliva following the same recommendations than at baseline. After collecting saliva, patients were instructed to place the vials inside a sealed bag containing absorbent paper in case of opening or breaking of saliva tube. Samples collection was supervised by investigator conducting the study visit.
The self-collection saliva vials contained solution used to inactivate the SARS-CoV-2 virus and stabilise viral nucleic acids at room temperature. After deactivation the saliva samples were stored at room temperature and then transferred to the Blizard Institute, Queen Mary University of London within 3 weeks for viral load analysis. Subsequently, samples were stored in a − 80 °C freezer until destruction in accordance with local guidelines and following the Human Tissue Authority (HTA) Code of Practice.
Mouthwash use
In the test groups (Group 1–3) patients also received as part of the self-test kit, mouthwash bottles. Immediately after baseline saliva collection, participants were instructed to vigorously rinse their mouth with 10 ml of Corsodyl Alcohol free (Group 1; 0.2% CHX), Colgate Peroxyl (Group 2; 1.5% H2O2) or Oral-B Gum & Enamel Care (Group 3; CPC) mouthwashes for 1 min, as per randomization. During rinsing, participants were asked to not gargle or swallow the mouthwashes.
Meanwhile, participants in the Group 4 (non-rinsing) were instructed to not rinse their mouth with any solution, not even water. All patients were allowed to drink water as needed during the study period up to 30 min prior each saliva collection.
Relative quantification of SARS-CoV-2 viral load using RT-qPCR
Saliva samples were thawed at room temperature and prior to nucleic acid extraction, saliva samples were spiked with Internal Extraction Control RNA (IEC; an exogenous RNA of rat Phogrin gene, NM_031600, amplicon 98 bp) from the genesig COVID-19 qPCR Assay kit (PrimerDesign Ltd., UK) to verify the successful extraction in case of a negative SARS-CoV-2 result (Fig. 2). Saliva samples provided by healthy volunteers were included as negative controls.