Abstract
The present study aimed to investigate the possible use of a non-instrumentation technique including blue light irradiation for root canal cleaning. Extracted human single rooted teeth were selected. Nine different groups included distilled water, NaOCl, intra-canal heated NaOCl, and NaOCl + EDTA irrigation after either instrumentation or non-instrumentation, and a laser application group following non-instrumentation technique. The chemical assessment of the root canal dentine was evaluated using energy dispersive spectroscopy (EDS) and Fourier transform infrared (FT-IR) spectroscopy. Surface microstructural analyses were performed by using scanning electron microscopy (SEM). The antimicrobial efficacy of different preparation techniques was evaluated using microbial tests. Light application didn’t change the calcium/phosphorus, carbonate/phosphate and amide I/phosphate ratios of the root canal dentin. The root canal dentin preserved its original chemistry and microstructure after light application. The instrumentation decreased the carbonate/phosphate and amide I/phosphate ratios of the root canal dentin regardless of the irrigation solution or technique (p < 0.05). The application of light could not provide antibacterial efficacy to match the NaOCl irrigation. The NaOCl irrigation both in the non-instrumentation and instrumentation groups significantly reduced the number of bacteria (p < 0.05). The use of minimally invasive root canal preparation techniques where the root canal is not instrumented and is disinfected by light followed by obturation with a hydraulic cement sealer reduced the microbial load and preserved the dentin thus may be an attractive treatment option for management of vital teeth needing root canal therapy.
Introduction
Root canal shaping and irrigation procedures are considered the most important steps in endodontic treatment1. The main aim of these procedures is the removal of necrotic tissue, microorganisms, and infected dentin from the root canal system1. For this reason, numerous root canal instrumentation and irrigation techniques, instruments, and devices have been proposed to effectively eliminate intra-canal debris and pathogens and manage apical periodontitis1,2,3,4,5,6,7,8.
Root canal instrumentation alone is unable to clean the root canal system adequately especially in the apical canal region9. Thus, root canal irrigation is a mandatory step during root canal treatment, as it enables the cleaning of parts of the root canal which are left untouched after mechanical instrumentation. Root canal instrumentation may result in procedural errors such as ledging, apical transportation, instrument fracture, elbow formation, and strip perforation10. Some complications such as extrusion of the irrigant or necrotic infected debris into the periapical area, air emphysema, splash of the irrigant into the operator’s or patient’s eye, and allergic reactions to the irrigant may occur during the irrigation of the root canals11. Moreover, instrumentation and irrigation procedures alter the surface morphology and the chemical and mechanical properties of the root canal dentin12,13,14.
Instrumentation of the root canals results in the formation of a smear layer that contains pulp tissue remnants, bacteria, infected dentin, and dead bacterial cells. It is well known that bacteria may survive and proliferate into the dentinal tubules in the presence of a smear layer15. Additionally, it may limit the optimum penetration of antibacterial solutions and can compromise adequate sealing by acting as a barrier between the root canal wall and filling materials16. It is possible to remove the smear layer from the root canal walls by using irrigation solutions that are primarily calcium chelators. However, irrigation procedures have some deleterious effects on root canal walls such as a decrease in the modulus of elasticity, microhardness, flexural strength, inorganic content, and the organic–inorganic ratio of the dentin17. These changes may jeopardise the success of root canal treatment via decreasing the fracture strength of root canal treated teeth. Therefore, the investigation of innovative root canal cleaning techniques, materials, and devices continues.
Cleaning of the root canal system without instrumentation and the creation of a smear layer may be possible with non-instrumentation techniques. Several studies evaluated different non-instrumentation techniques and reported promising results18,19,20. However, all these studies used NaOCl irrigation in the non-instrumentation groups for the disinfection of the root canals, which, has numerous deleterious effects on root canal dentin17. Therefore, a minimally invasive root canal cleaning technique that does not include instrumentation and chemical irrigation of the root canals is necessary.
This study proposes an alternative antimicrobial approach to root canal decontamination using the direct application of blue light in the spectrum of 400–470 nm which has been reported to have antibacterial effects against a variety of pathogens8. Previous studies.8 evaluated the antimicrobial efficacy of blue light and demonstrated that its application significantly eliminated endodontic pathogens such as Enterococcus faecalis, methicillin-resistant Streptococcus aureus, and Prevotella intermedia. The blue light exerts its antibacterial effect by excitation of endogenous microbial chromophores such as flavins and porphyrins which subsequently results in the generation of reactive oxygen species (ROS)7. Several studies demonstrated that direct light application is useful for the disinfection of dental tissues and can inhibit a wide range of bacterial species residing within biofilms7,8,21,22,23. Microorganisms also do not become resistant to blue light in the long term 24. However, the antimicrobial effects of blue light depend on several factors, namely the irradiance of the light and the exposure duration which determine the energy delivered to the target site. The delivery of light for intra-canal irradiation is complicated by the complex architecture and microstructure of dental tissues which will absorb and scatter light to limit the amount of light that can be delivered to microorganisms for effective antimicrobial effects. Currently, it is not known if the direct application of light can obtain adequate bacterial reduction after intra-canal irradiation. Additionally, the effect of direct light application on the chemical and mechanical properties of dental tissues including the root canal dentin has not been well established. The present study aimed to investigate the possible use of a non-instrumentation technique including blue light irradiation for root canal cleaning without instrumentation and chemical irrigation followed by obturation using a hydraulic cement sealer for the management of vital teeth requiring root canal therapy. Thus, the deleterious effects of instrumentation and irrigation procedures on root canal dentin would be excluded. This methodology was compared to standard root canal instrumentation and irrigation protocols and obturation with gutta-percha and sealer.
Results
Light transmission and irradiance measurement
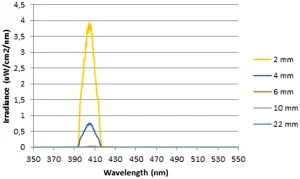
Light transmission measurements revealed that the irradiances delivered to the lower surfaces of 2, 4, 6, 10, and 22-mm samples for the highest power settings were 3.99, 0.55, 0.04, 0.01, and 0.002 mW/cm2 respectively (Fig. 1). The light transmission was negligible in samples with a thickness of more than 4 mm. Therefore, it has been decided that both intra-canal and extra-canal applications of light should be considered to able to irradiate whole the root canal. For this reason, laser fibre irradiance measurements were performed. According to the results, in the highest current setting of the laser device, the irradiance was 38,072 and 1032 mW/cm2 for 105 and 200 µm fibres, respectively.
Analysis of the heat changes
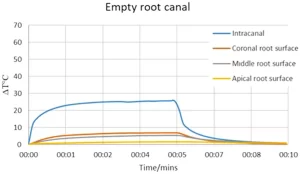
The intra-canal and external root surface heat changes during the laser application with 200 µm are shown in Fig. 2. The temperature inside the root canal increased immediately with the application of the laser and reached a maximum rise of 59 °C when the root canal was empty. However, the maximum temperature rise recorded during the laser application with the presence of distilled water inside the root canal was only 27 °C. Similarly, while the highest increase in temperature on the external root surface was 10.8 °C in the empty root canal, it was only 5.9 °C in the sample the root canal was filled with distilled water. The highest temperature change on the external root surface was recorded at the middle root level as the tip of the laser fibre was at 5 mm level from the apex.

Since the increase in temperature in the empty root canal sample was higher than the biological limits for outer surface of the root canal (10 °C), it has been decided to apply the laser (200 µm fibre) inside the root canal with the presence of distilled water throughout the study.
The temperature rise in the laser application from the root canal orifice can be seen in Fig. 3. The maximum increase in temperature was 25 °C and 6.8 °C for intra-canal and external root surface measurements respectively. The highest change in temperature on the external root surface (6.8 °C) was observed at the coronal level. Since the maximum temperature changes were within the biological limits, it has been decided that filling the root canal with distilled water is not necessary and it is safe to apply the laser (105 µm fibre) from the root canal orifice while the root canal was dry and empty.
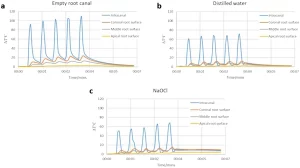
The temperature changes during the intra-canal application of heat with SuperEndo can be seen in Fig. 4. In all the samples, the temperature both inside the root canal and external root surface increased immediately with the activation of the heat source, and an immediate decrease was also observed once the heat source was deactivated. However, the maximum rise in the temperature inside the root canal was 109 °C and 23.7 °C for intra-canal and external root surface measurements in the empty root canal sample. In contrast, the maximum intra-canal temperature rises were 71 °C and 67 °C for the distilled water and NaOCl samples respectively. External root surface temperature change measurements were also relatively similar for the distilled water and NaOCl samples. The maximum temperature rise on the external root surface was 12.2 °C and 14.4 °C for the distilled water and NaOCl samples respectively. It has been demonstrated that the temperature rises were above the biological limits for all the 3 samples. Most importantly, the temperature of the root surface could not be restored for 2 min following the completion of the 5 cycles of the irrigation procedure, especially in distilled water and NaOCl samples.
SEM evaluation
Figure 5 shows the SEM images for the groups. The SEM images showed that root canal dentin preserved its original form in the light application group without any damage as in the non-instrumentation distilled water group. After root canal instrumentation, the dentin surface was covered with a smear layer which the distilled water and NaOCl irrigation were unable to remove. Final irrigation with EDTA removed the smear layer and presented clean walls with opened and widened dentinal tubules. Although instrumentation was not performed in the non-instrumentation groups, a smear layer was formed in the NaOCl and heated NaOCl groups. This erosive effect was more evident in the non-instrumentation heated NaOCl group, showing a tunnelling erosion pattern on the dentin, with enlarged, interconnected dentinal tubular spaces. Final irrigation with EDTA clearly showed the loss of integrity of the inter-tubular dentin.